Made a visit to Jack's yard today while I was there cut these silver soldered copper ends off of a few industrial condensers, with silver now at $27.70 a troy ounce its worth chasing.
After burning off the oil residue the ends get melted into a cathode, the tube video below pretty much explains the rest of the process.
From Ammen's book Refining Precious Metals Wastes.
The crude copper is the anode, and a pure copper starting sheet is the cathode. The electrolyte is made by dissolving, in distilled water, copper sulphate (CuSOJ, a copper salt that is approximately 25% copper. Some sulphuric acid is added as free acid to increase the
conductivity of the bath.
A typical copper-refining acid bath, in miniature, would be: 250 grams per liter of copper sulphate, and 75 grams per liter of sulphuric acid; a bath operating temperature of 70° to 120°F; a voltage of 6 to 8 volts; and a current density in amperes of from 20 to 100 amperes
per square foot of cathode surface.
Copper from the crude copper anode goes into solution and the positive ions of pure copper ( C u ) are carried over to the cathode by electromotive force through the electrolyte, where they are neutralized










 Register To Reply
Register To Reply

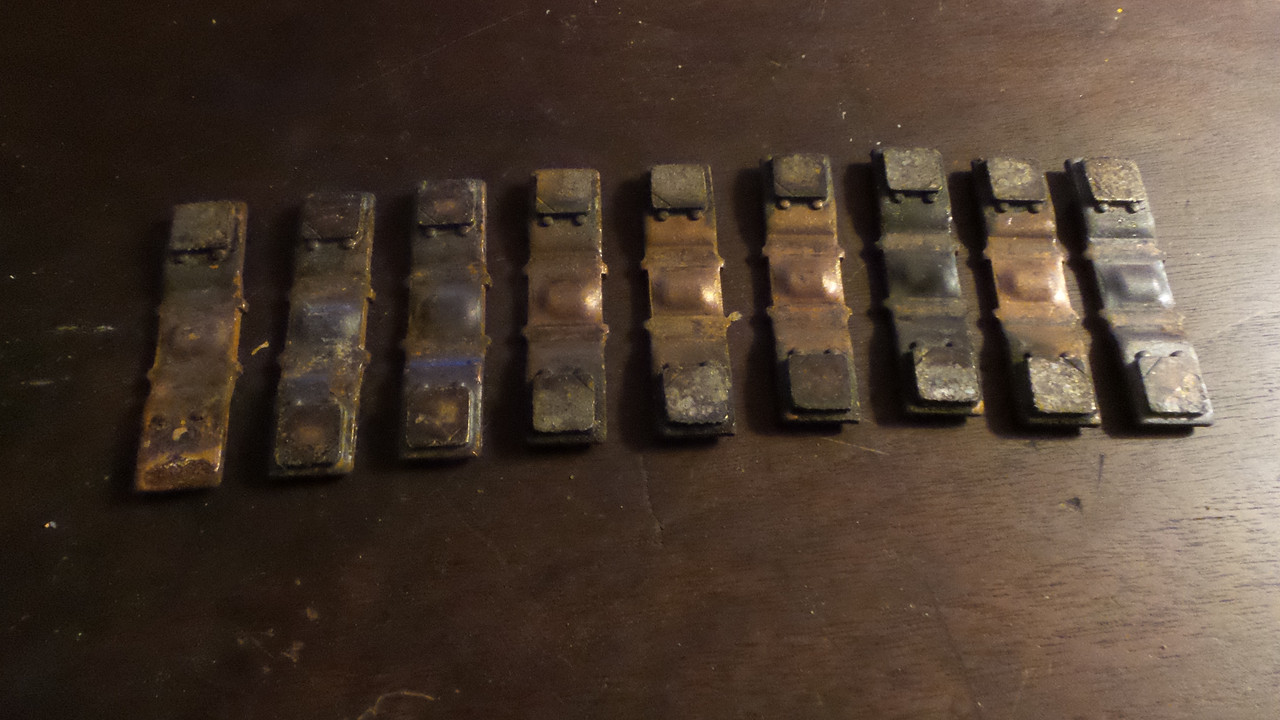























Bookmarks